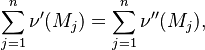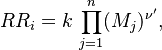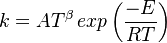Combustion
From CFD-Wiki
(→Reaction mechanisms) |
(→References) |
||
| Line 90: | Line 90: | ||
== References == | == References == | ||
| + | |||
| + | <div id="Griffiths"> | ||
| + | {{reference-paper|author=Griffiths J.F.|year=1994|title=Reduced Kinetic Models and Their Application to Practical Combustion Systems |rest=Prog. in Energy and Combustion Science,Vol. 21, pp. 25-107}} | ||
| + | </div> | ||
| + | <div id="Westbrook"> | ||
| + | {{reference-paper|author=Westbrook, Ch.K., Dryer,F.L.,|year=1984|title=Chemical Kinetic Modeling of Hydrocarbon Combustion |rest=Prog. in Energy and Combustion Science,Vol. 10, pp. 1-57}} | ||
| + | </div> | ||
| + | |||
== External links and sources == | == External links and sources == | ||
Revision as of 10:32, 18 September 2005
Contents |
What is combustion -- Physics versus modelling
Combustion phenomena consists of many physical and chemical processes with broad range of time scales. Mathematical description of combustion is not always trivial. Analytical solutions exists only for basic situations of laminar flame and because of its assumptions it is often restricted to few problems solved usually in zero or one-dimensional space.
Problems solved today concern mainly turbulent flows, gas as well as liquid fuels, pollution issues (products of combustion as well as for example noise pollution). These problems require not only extensive experimental work, but also numerical modelling. All combustion models must be validated against the experiments as each one has its own drawbacks and limits. However here the modelling part will be mainly addressed.
Reaction mechanisms
The combustion is mainly chemical process and although we can, to some extend, describe flame without any chemistry informations, for modelling of flame propagation we need to know the speed of reactions, product concentrations, temperature and other parameters. Therefore more or less detailed information about reaction kinetics is essential for any combustion model. \par Mixture will generally combust, if the reaction of fuel and oxidiser is fast enough to maintain until all of the mixture is burned into products. If the reaction is too slow, the flame will extinguish, if too fast, explosion or even detonation will occur. The reaction rate of typical combustion reaction is influenced mainly by concentration of reactants, temperature and pressure.
A stoichiometric equation of an arbitrary equation can be written as:
|
| (2.1) |
where $\nu$ is the stoichiometric coefficient, 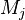 is arbitrary species. One
prime specifies the reactants and double prime products of the reaction.
is arbitrary species. One
prime specifies the reactants and double prime products of the reaction.
Reaction rate, expressing the rate of disappearance of reactant i of such a reaction, is defined as:
|
| (2.2) |
in which k is the specific reaction rate constant. Arrhenius found that this constant is a function only of temperature and this function is defined as:
|
| (2.3) |
where A is pre--exponential factor, E is activation energy and 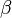 is
temperature exponent.
These constants for given reactions can be found in literature.
The reaction mechanism can be given from experiments for every reaction
resolved, it could be also constructed numerically by automatic generation
method (see article of #Griffiths for review on reaction mechanisms).
For simple hydrocarbon tens to hundreds of reactions are involved.
By analysis and systematic reduction of reaction mechanisms global reaction
(from one to five step reactions) can be found (see #Westbrook).
is
temperature exponent.
These constants for given reactions can be found in literature.
The reaction mechanism can be given from experiments for every reaction
resolved, it could be also constructed numerically by automatic generation
method (see article of #Griffiths for review on reaction mechanisms).
For simple hydrocarbon tens to hundreds of reactions are involved.
By analysis and systematic reduction of reaction mechanisms global reaction
(from one to five step reactions) can be found (see #Westbrook).
Infinitely fast chemistry
Turbulent flame speed model
Eddy Break-Up model
Equilibrium chemistry models
Finite chemistry
Full reaction mechanism model
Reduced scheme models
- Flamelet model
- Other reaction progress variable models
References
Griffiths J.F. (1994), "Reduced Kinetic Models and Their Application to Practical Combustion Systems", Prog. in Energy and Combustion Science,Vol. 21, pp. 25-107.
Westbrook, Ch.K., Dryer,F.L., (1984), "Chemical Kinetic Modeling of Hydrocarbon Combustion", Prog. in Energy and Combustion Science,Vol. 10, pp. 1-57.