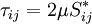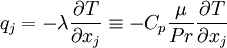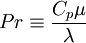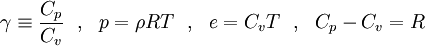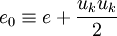Favre averaged Navier-Stokes equations
From CFD-Wiki
| Line 1: | Line 1: | ||
| - | + | == Instantaneuos Equations == | |
| - | + | ||
| - | + | ||
| + | The instantaneous continuity equation (1), momentum equation (2) and energy equation (3) for a compressible fluid can be written as: | ||
| + | |||
| + | <table width="100%"> | ||
| + | <tr><td> | ||
:<math> | :<math> | ||
\frac{\partial \rho}{\partial t} + | \frac{\partial \rho}{\partial t} + | ||
\frac{\partial}{\partial x_j}\left[ \rho u_j \right] = 0 | \frac{\partial}{\partial x_j}\left[ \rho u_j \right] = 0 | ||
| - | </math> (1) | + | </math> |
| - | + | </td><td width="5%">(1)</td></tr> | |
| + | <tr><td> | ||
:<math> | :<math> | ||
\frac{\partial}{\partial t}\left( \rho u_i \right) + | \frac{\partial}{\partial t}\left( \rho u_i \right) + | ||
\frac{\partial}{\partial x_j} | \frac{\partial}{\partial x_j} | ||
\left[ \rho u_i u_j + p \delta_{ij} - \tau_{ji} \right] = 0 | \left[ \rho u_i u_j + p \delta_{ij} - \tau_{ji} \right] = 0 | ||
| - | </math> (2) | + | </math> |
| - | + | </td><td>(2)</td></tr> | |
| + | <tr><td> | ||
:<math> | :<math> | ||
\frac{\partial}{\partial t}\left( \rho e_0 \right) + | \frac{\partial}{\partial t}\left( \rho e_0 \right) + | ||
\frac{\partial}{\partial x_j} | \frac{\partial}{\partial x_j} | ||
\left[ \rho u_j e_0 + u_j p + q_j - u_i \tau_{ij} \right] = 0 | \left[ \rho u_j e_0 + u_j p + q_j - u_i \tau_{ij} \right] = 0 | ||
| - | </math> (3) | + | </math> |
| + | </td><td>(3)</td></tr> | ||
| + | </table> | ||
| - | For a Newtonian fluid, assuming Stokes Law for mono-atomic gases, the viscous | + | For a Newtonian fluid, assuming Stokes Law for mono-atomic gases, the viscous stress is given by: |
| - | stress is given by: | + | |
| - | <math> | + | <table width="100%"> |
| + | <tr><td> | ||
| + | :<math> | ||
\tau_{ij} = 2 \mu S_{ij}^* | \tau_{ij} = 2 \mu S_{ij}^* | ||
</math> | </math> | ||
| + | </td><td width="5%">(4)</td></tr> | ||
| + | </table> | ||
| - | Where the trace-less viscous strain-rate is defined | + | Where the trace-less viscous strain-rate is defined by: |
| - | by: | + | |
| - | <math> | + | <table width="100%"> |
| + | <tr><td> | ||
| + | :<math> | ||
S_{ij}^* \equiv | S_{ij}^* \equiv | ||
\frac{1}{2} \left(\frac{\partial u_i}{\partial x_j} + | \frac{1}{2} \left(\frac{\partial u_i}{\partial x_j} + | ||
| Line 36: | Line 46: | ||
\frac{1}{3} \frac{\partial u_k}{\partial x_k} \delta_{ij} | \frac{1}{3} \frac{\partial u_k}{\partial x_k} \delta_{ij} | ||
</math> | </math> | ||
| + | </td><td width="5%">(5)</td></tr> | ||
| + | </table> | ||
The heat-flux, <math>q_j</math>, is given by Fourier's law: | The heat-flux, <math>q_j</math>, is given by Fourier's law: | ||
| - | <math> | + | <table width="100%"> |
| + | <tr><td> | ||
| + | :<math> | ||
q_j = -\lambda \frac{\partial T}{\partial x_j} | q_j = -\lambda \frac{\partial T}{\partial x_j} | ||
\equiv -C_p \frac{\mu}{Pr} \frac{\partial T}{\partial x_j} | \equiv -C_p \frac{\mu}{Pr} \frac{\partial T}{\partial x_j} | ||
</math> | </math> | ||
| + | </td><td width="5%">(6)</td></tr> | ||
| + | </table> | ||
| - | Where the laminar Prandtl number <math>Pr</math> is defined | + | Where the laminar Prandtl number <math>Pr</math> is defined by: |
| - | by: | + | |
| - | <math> | + | <table width="100%"> |
| + | <tr><td> | ||
| + | :<math> | ||
Pr \equiv \frac{C_p \mu}{\lambda} | Pr \equiv \frac{C_p \mu}{\lambda} | ||
</math> | </math> | ||
| + | </td><td width="5%">(7)</td></tr> | ||
| + | </table> | ||
| - | To close these equations it is also necessary to specify an equation of state. | + | To close these equations it is also necessary to specify an equation of state. Assuming a calorically perfect gas the following relations are valid: |
| - | Assuming a calorically perfect gas the following relations are valid: | + | |
| - | <math> | + | <table width="100%"> |
| + | <tr><td> | ||
| + | :<math> | ||
\gamma \equiv \frac{C_p}{C_v} ~~,~~ | \gamma \equiv \frac{C_p}{C_v} ~~,~~ | ||
p = \rho R T ~~,~~ | p = \rho R T ~~,~~ | ||
| Line 60: | Line 80: | ||
C_p - C_v = R | C_p - C_v = R | ||
</math> | </math> | ||
| + | </td><td width="5%">(8)</td></tr> | ||
| + | </table> | ||
| - | Where <math>\gamma, C_p, C_v</math> and <math>R</math> are constant. | + | Where <math>\gamma</math>, <math>C_p</math>, <math>C_v</math> and <math>R</math> are constant. |
The total energy <math>e_0</math> is defined by: | The total energy <math>e_0</math> is defined by: | ||
| - | <math> | + | <table width="100%"> |
| + | <tr><td> | ||
| + | :<math> | ||
e_0 \equiv e + \frac{u_k u_k}{2} | e_0 \equiv e + \frac{u_k u_k}{2} | ||
</math> | </math> | ||
| + | </td><td width="5%">(9)</td></tr> | ||
| + | </table> | ||
| - | Note that the | + | Note that the corresponding expression <table><tr><td bgcolor="red">Insert Reference</td></tr></table> for Favre averaged turbulent flows contains an extra term related to the turbulent energy. |
| - | corresponding expression | + | |
| - | for Favre averaged turbulent flows contains an | + | |
| - | extra term related to the turbulent energy. | + | |
| + | Equations (1)-(9), supplemented with gas data for <math>\gamma</math>, <math>Pr</math>, <math>\mu</math> and perhaps <math>R</math>, form a closed set of partial differential equations, and need only be complemented with boundary conditions. | ||
| + | == | ||
Revision as of 08:36, 5 September 2005
Instantaneuos Equations
The instantaneous continuity equation (1), momentum equation (2) and energy equation (3) for a compressible fluid can be written as:
|
| (1) |
|
| (2) |
|
| (3) |
For a Newtonian fluid, assuming Stokes Law for mono-atomic gases, the viscous stress is given by:
|
| (4) |
Where the trace-less viscous strain-rate is defined by:
|
| (5) |
The heat-flux,  , is given by Fourier's law:
, is given by Fourier's law:
|
| (6) |
Where the laminar Prandtl number  is defined by:
is defined by:
|
| (7) |
To close these equations it is also necessary to specify an equation of state. Assuming a calorically perfect gas the following relations are valid:
|
| (8) |
Where 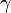 ,
, 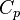 ,
,  and
and  are constant.
are constant.
The total energy 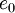 is defined by:
is defined by:
|
| (9) |
| Insert Reference |
Equations (1)-(9), supplemented with gas data for 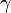 ,
,  ,
, 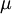 and perhaps
and perhaps  , form a closed set of partial differential equations, and need only be complemented with boundary conditions.
, form a closed set of partial differential equations, and need only be complemented with boundary conditions.
==
![\frac{\partial \overline{\rho}}{\partial t} +
\frac{\partial}{\partial x_i}\left[ \overline{\rho} \widetilde{u_i} \right] = 0](/W/images/math/d/c/d/dcd521718edfb790fcf5915dfff51dde.png)
![\frac{\partial}{\partial t}\left( \overline{\rho} \widetilde{u_i} \right) +
\frac{\partial}{\partial x_j}
\left[
\overline{\rho} \widetilde{u_j} \widetilde{u_i}
+ \overline{p} \delta_{ij}
- \widetilde{\tau_{ji}^{tot}}
\right]
= 0](/W/images/math/c/9/f/c9fb98d7716cc9a91f7e78b0ed597728.png)

![\frac{\partial \rho}{\partial t} +
\frac{\partial}{\partial x_j}\left[ \rho u_j \right] = 0](/W/images/math/0/7/1/071e4f5508fd336ddad848551ce3188e.png)
![\frac{\partial}{\partial t}\left( \rho u_i \right) +
\frac{\partial}{\partial x_j}
\left[ \rho u_i u_j + p \delta_{ij} - \tau_{ji} \right] = 0](/W/images/math/6/f/c/6fc8041faa4be98ee72ec1e670fb22c7.png)
![\frac{\partial}{\partial t}\left( \rho e_0 \right) +
\frac{\partial}{\partial x_j}
\left[ \rho u_j e_0 + u_j p + q_j - u_i \tau_{ij} \right] = 0](/W/images/math/8/1/7/8176cdf87a72617542883cbbeafc50cc.png)